Learn More
Epilepsy - The Sacred Disease
Epilepsy is a condition of the brain, characterised by abnormal patterns of electrical activity that build up and interfere with brain function. These sudden and recurrent episodes are called seizures. In fact, the word Epilepsy comes from Greek meaning “to be seized upon”.
Although the ancient Greek physician Hippocrates suggested a physiological basis for seizures, the historical treatment of epilepsy was largely based on spiritual beliefs. The first documented record of a seizure— in around 2000 BC —was attributed to the influence of a moon god. Since then epilepsy has been connected with spiritual realms, and suggestions of possession by higher powers led to the condition being known as the ‘Sacred Disease’. However, despite this name, the history of epilepsy is one largely of persecution and exclusion for sufferers. Even today, there are common misperceptions about the condition. For example, in a recent study, over half of respondents incorrectly believed that one should put something in the mouth of a person having a seizure to prevent them from biting or swallowing their tongue.

Epilepsy is a very broad condition. Symptoms vary depending on the type of epilepsy and area of brain affected, meaning every person with epilepsy experiences the condition slightly differently. Some people may simply have an odd feeling or be in a “trance-like” state for a few seconds or minutes. Others may be more severely affected, losing control of their body and even consciousness. Because the occurrence of seizures is unpredictable, epilepsy can have a big impact on people’s ability to enjoy life to the fullest.
It is not always possible to identify the reason someone develops epilepsy. Some cases may result from faulty or damaged DNA (the genetic code used to build the body). Other causes include damage to the brain following a stroke or severe head injury. While epilepsy can affect people of all ages, it is more commonly found in children and older adults.
The treatment of epilepsy has been transformed over the last century by the development of anti-epileptic drugs. While these drugs cannot cure epilepsy, they are effective in controlling seizures for around 70% of people. Surgery to remove the part of the brain responsible for seizures provides another option for some, but many brain areas cannot be removed without serious side effects. There is, therefore, an ongoing need to discover new therapies for treating epilepsy.
One possibility is to use electrical stimulation to treat epilepsy, similar to Deep Brain Stimulation therapy that has proved effective for Parkinson’s disease. Several international research groups are exploring whether this can also prevent seizures. The CANDO project is taking a new approach exploring the use of light to treat epilepsy. But how exactly can light help control seizures?
Optogenetics – A new dawn for treating disease?
In 1979, Francis Crick, one of the scientists attributed with discovering the double helix structure of DNA, said the greatest challenge facing neuroscientists was the need to control and manipulate one type of brain cell whilst leaving the others undisturbed. Solving this challenge would help explain the roles of different types of brain cells, something that the current electrical techniques are unable to achieve.
The answer has come in the form of ‘optogenetics’, a type of gene therapy involving naturally occurring light-sensitive proteins called opsins. These proteins, which can be found across all types of life, change shape when illuminated with light. In fact, it is the opsins in our eyes that allow us to see. There are many types of opsins and some form channels or pumps between the inside and the outside of cells. Shining light on these opsins causes charged atoms, or ions, to move in and out of the cells. The flow of charged ions generates electrical currents, which play a key role in how brain cells communicate with one another. Opsins can be used to turn on or off communication, and thereby influence patterns of brain activity.
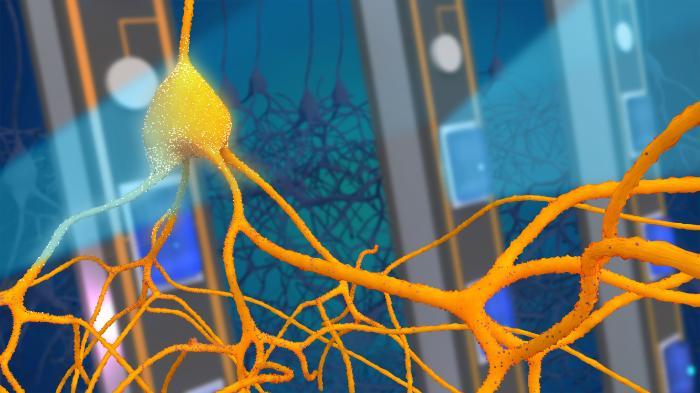
One advantage of using light, rather than drugs, to control brain activity is that light can be turned on only when needed. By simultaneously recording electrical signals associated with seizures, light can be delivered at precisely the right moments to achieve optimal therapeutic effect. This is known as ‘closed-loop stimulation’ because the brain signals control the light, which in turn controls the brain in a continuous feedback loop. A common example of a feedback loop is a thermostat, which only turns the heating on when necessary and thereby stabilises the temperature of a room. Similarly, by applying feedback to the brain using optogenetics, we can stabilise brain activity and prevent seizures developing.
Gene Therapies and Viral Vectors
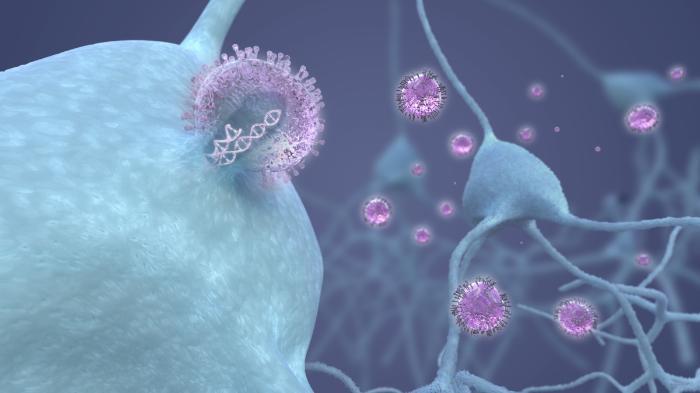
Although opsins in our eyes allow us to see, they do not naturally occur within the brain. To use opsins to treat epilepsy, we need a way to introduce them safely into specific cells in the brain. This is now possible with gene therapy technology.
As early as 1966, scientists recognised that viruses possessed properties that could be useful in delivering genes into cells. A virus is a small infectious agent that requires another living cell to replicate. Viruses enter cells and instruct the cell’s own machinery to make new copies of itself, causing the infection to spread. By contrast, the modified viral vector in a gene therapy makes copies of useful proteins like opsins. It does this by introducing a new piece of genetic code into the cell, which contains the instructions for making the opsin. Importantly, this code does not contain instructions for making more virus, and therefore the viral vector is not infectious and cannot spread. Moreover, genetic material inserted only into brain cells does not get passed on to any future children. Optogenetic gene therapies are already being trialled in humans, delivered to the eye as a potential treatment for blindness. But before any optogenetic therapy is tested in the human brain, it is essential to prove that the viral vector and opsin proteins are not harmful.
Engineering for the brain
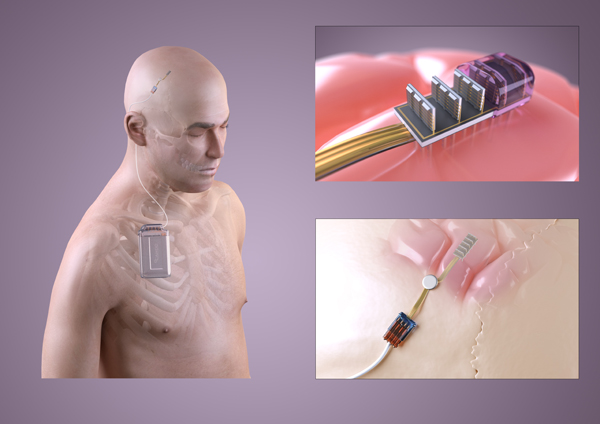
Alongside the gene therapy, an optogenetic treatment for epilepsy also requires an implant in the brain. This implant must detect abnormal electrical activity and deliver the appropriate light stimulation. One of the main challenges is developing an implant small enough to fit within the skull. Moreover, the right materials must be used so that water cannot get in and cause the implant to fail. The implant must be powered, requiring a battery that can be recharged periodically through the skin. There also needs to be a way for doctors to calibrate the settings and check the implant is working correctly. Because brain surgery is needed to place the implant, it is critical that it carries on working for many years. It is therefore vital to thoroughly test all aspects of the device before using in humans. These important preclinical tests are currently being undertaken in the CANDO project.
Ethical Implications
What does it mean to have a device implanted into the body? What are the implications of introducing new genetic code into our cells? How do therapies that alter our brain function affect our sense of being human?
Technology is increasingly making incursions into our lives and our bodies. Replacement limbs or prosthetics have been around for millennia, but pacemakers now restore heart function, insulin pumps control diabetes and cochlear implants help the deaf to hear. Do brain implants raise further ethical concerns? Is it right to collect data from the brain and who should this information belong to? If optogenetics can prevent seizures in the brain, could it in future alter behaviour or personality? Can we be sure that brain implants will not be hacked for malicious purposes? Ex vice-president of the USA, Dick Cheney, disabled communication to his pacemaker for fear it might be hacked to induce a heart attack – imagine the concerns over an implant that could affect his thoughts!
At present, gene therapy and brain implant research is focussed on restoring healthy function in medical conditions like epilepsy. But as these technologies advance, we may be able to improve our capabilities above and beyond normal levels, for example enhancing our memory or attention. Where do the ethical boundaries lie in regards to genetic and neurological enhancement? Companies such as Facebook and Elon Musk’s Neuralink are already investing in neurotechnology to enhance our interaction with computers. Who gets access to such enhancements, and who gets to decide the limits of our bioengineered future?
These are not decisions that can be left solely to scientists, engineers, doctors or even patients. As a project we have been exploring these conversations through our public engagement work 'Illuminating the Self'.
