

Project Timeline
CANDO kicked off in August 2014. Over seven years the project will progress through several phases. Initial phases focus on technology design and development, followed by rigorous testing of performance and safety. Due to the COVID-19 pandemic, the project timeline has been extended until 2022.
The project has recently passed its sixth milestone in the project. Significant progress has been made in identifying the optimal design for elements of the device and the selection of potential vectors for delivering the gene therapy. We are now moving forward with testing prototypes of the implantable device and the gene therapy for safety and efficacy.
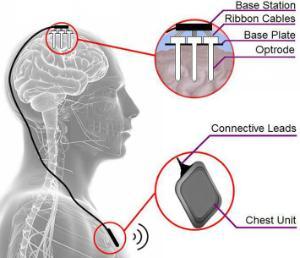
We are now reporting our findings at conferences, symposiums and in journal papers. Click on the Publications tab at the top to look through our published papers.
